One promising area of research in cancer therapy is the
development of theranostics. This area of research focuses on simultaneous
application of both a therapeutic agent (typically a chemotherapeutic agent
such as paclitaxel) and a diagnostic agent (typically a contrast agent or fluorescent
dye which renders the tumor ‘visible’). This research requires highly advanced
delivery systems which can ensure that the tumor receives a suitable quantity
of both agents such that it becomes visible to a surgeon as well as receives an
effective dose of the therapeutic agent. In a fundamental sense, this requires
well-designed nanocarriers with high loading efficiency (large doses of each
agent) and which are highly stable in the bloodstream. Recently, researchers at
Wroclaw University (Poland) utilized a series of PolySciTech (www.polyscitech.com) polymers including
mPEG-PCL (PolyVivo Cat# AK128), mPEG-PLA (PolyVivo Cat# AK056), and mPEG-PLGA
(PolyVivo Cat# AK037) to systematically generate a series of test-loaded
nanoparticles containing model DNA and fluorescent dye Thiazole Orange. The
researchers systematically investigated all steps involved in nanoparticle
formation and tested the particles for their stability, loading capacity, and
other parameters relevant to their clinical usage. This research holds promise
for the development of highly advanced nanocarriers to assist in theranostic
treatments of a wide variety of cancers. Read more: Bazylińska, Urszula. "Rationally designed
double emulsion process for co-encapsulation of hybrid cargo in stealth
nanocarriers." Colloids and Surfaces A: Physicochemical and Engineering
Aspects (2017). http://www.sciencedirect.com/science/article/pii/S092777571730359X
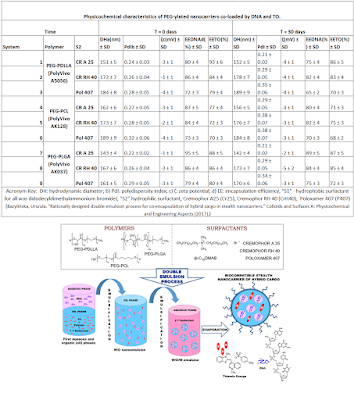
“Abstract: Double emulsion process has become
highly promising for development of PEG-ylated nanocarriers (NCs) with
co-encapsulated hybrid model agents, i.e, hydrophilic deoxyribonucleic acid
(DNA) and hydrophobic Thiazole Orange (TO) dye, in the double compartment
structure to protect them from the environmental conditions and to investigate
different parameters affecting the size, charge and morphology as well as
colloidal and biological stability of the final theranostic nanosystems.
Different stabilizing agents including surfactants: Cremophor A25, Cremophor RH
40, Poloxamer 407, di-C12DMAB as well as polymers: PEG-PDLLA, PEG-PLGA,
PEG-PCL, were screened to choose suitable ones for this approach. The average
size of the synthesized NCs measured by dynamic light scattering (DLS) remained
< 200 nm. The encapsulation efficiency of the hybrid cargo was confirmed by
UV-Vis spectroscopy. Morphology and shape of the loaded nanocontainers were
investigated by transmission electron microscopy (TEM) and atomic force
microscopy (AFM). Time-depended colloidal stability studies with DLS and
ζ-potential followed by turbidimetric technique allow to select only the
long-term nanosystems to final investigation the “stealth” properties of the
fabricated PEGylated NCs. Highlights: Double emulsion process has become
easy-scalable synthetic approach to develop “stealth” nanocarriers (NCs)
successful in DNA and TO co-encapsulation. PEG-PDLLA, PEG-PLGA, PEG-PCL acted
as pre-approved biocompatible components of the NCs polymer shell.The optimized
encapsulation process resulted in NCs with diameter < 200 nm, narrow size
distribution and nearly neutral surface. DLS, ζ-potential and backscattering
studies confirmed a long-term NCs stability, indicating their potential as
theranostic biocompatible agents. The biological stability exposed the
PEG-ylated NCs ability to overcome various specific barriers to efficient drug
and gene delivery. Keywords: w/o/w emulsions; PEG-ylated polyesters; DNA;
Thiazole Orange; colloidal stability. Fabrication method: Polymeric
nanocarriers stabilized by PEG-PLGA, PEG-PCL, PEG-PDLLA and non-ionic or
cationic surfactants for co-encapsulation of therapeutic (model DNA in the
initial concentration of 0.1 mg/ml) and diagnostic agent (TO in the initial
concentration of 0.2 mg/ml) were prepared by modified double emulsion (w/o/w)
evaporation process without any pH adjustment [8]. Generally, aqueous internal
phase (with DNA) was emulsified for 5 min in dichloromethane (containing TO,
PEG-ylated polymer in concentration of 5 mg/ml and di-C12DMAB) in the ratio 1:4
using a homogenizer with 25,000 rpm. This primary nanoemulsion was poured into
1% hydrophilic surfactant solution (Cremophor A25, Cremophor RH 40 or Poloxamer
407) aqueous solution stirring in a homogenizer for 10 min (25,000 rpm) and
immersed in an ice water bath to create the water-in-oil-in-water (w/o/w)
emulsion. The organic solvent was then evaporated under reduced pressure in a
rotary evaporator (Ika RV 10 digital) and polymeric nanocarriers loaded by the
hybrid cargo were collected overnight.”
No comments:
Post a Comment