A powerful technique commonly applied in
tissue engineering is cell-scaffolding in which a highly-porous, biocompatible material
is implanted into a patient. This mesh allows for cells to grow inside of its
structure to repair damaged or lost tissue. There still remains a great deal to
learn about exactly how cells interact with the substrate they are growing on,
as the structure and chemistry of the mesh is critical to how the cells grow.
One common problem with growing roughly translucent microscopic cells on an
opaque microfiber mesh is visualizing what is going on with the cells. This is
where fluorescent imaging comes in to play. In this method, each component is
bound to a specific dye that emits light when excited by a specific wavelength
of light. This allows researchers to image each component of the system,
separately. Recently, researchers from National institute of Standards and
Technology (NIST) and National Institute of Health (NIH) utilized fluorescently
conjugated PLGA-FKR648 (PolyVivo AV015) from PolySciTech (www.polyscitech.com) to make a series of cell-scaffolds and test their
interactions with cells. The fluorescent nature of this polymer allowed for
direct imaging of the mesh by fluorescence techniques, so they could
investigate cell-interactions in fine detail. This research provides a valuable
tool for tissue-engineering researchers looking to optimize their mesh designs.
Read more: Bajcsy, Peter, Soweon Yoon, Stephen J. Florczyk, Nathan A. Hotaling,
Mylene Simon, Piotr M. Szczypinski, Nicholas J. Schaub, Carl G. Simon, Mary
Brady, and Ram D. Sriram. "Modeling, validation and verification of three-dimensional
cell-scaffold contacts from terabyte-sized images." BMC Bioinformatics 18,
no. 1 (2017): 526. https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-017-1928-x
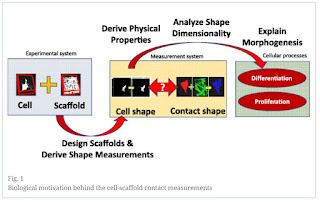
“Background: Cell-scaffold contact
measurements are derived from pairs of co-registered volumetric fluorescent
confocal laser scanning microscopy (CLSM) images (z-stacks) of stained cells
and three types of scaffolds (i.e., spun coat, large microfiber, and medium
microfiber). Our analysis of the acquired terabyte-sized collection is
motivated by the need to understand the nature of the shape dimensionality (1D
vs 2D vs 3D) of cell-scaffold interactions relevant to tissue engineers that
grow cells on biomaterial scaffolds. Results: We designed five statistical and
three geometrical contact models, and then down-selected them to one from each
category using a validation approach based on physically orthogonal
measurements to CLSM. The two selected models were applied to 414 z-stacks with
three scaffold types and all contact results were visually verified. A planar
geometrical model for the spun coat scaffold type was validated from atomic
force microscopy images by computing surface roughness of 52.35 nm ±31.76 nm
which was 2 to 8 times smaller than the CLSM resolution. A cylindrical model
for fiber scaffolds was validated from multi-view 2D scanning electron
microscopy (SEM) images. The fiber scaffold segmentation error was assessed by comparing
fiber diameters from SEM and CLSM to be between 0.46% to 3.8% of the SEM
reference values. For contact verification, we constructed a web-based visual
verification system with 414 pairs of images with cells and their segmentation
results, and with 4968 movies with animated cell, scaffold, and contact
overlays. Based on visual verification by three experts, we report the accuracy
of cell segmentation to be 96.4% with 94.3% precision, and the accuracy of
cell-scaffold contact for a statistical model to be 62.6% with 76.7% precision
and for a geometrical model to be 93.5% with 87.6% precision. Conclusions: The
novelty of our approach lies in (1) representing cell-scaffold contact sites
with statistical intensity and geometrical shape models, (2) designing a
methodology for validating 3D geometrical contact models and (3) devising a
mechanism for visual verification of hundreds of 3D measurements. The raw and
processed data are publicly available from
https://isg.nist.gov/deepzoomweb/data/ together with the web -based
verification system. Keywords: Co-localization Cellular measurements
Cell-scaffold contact Segmentation models Contact evaluation Web-based
verification Large-volume 3D image processing”
No comments:
Post a Comment