HPV is a common STD which leads to cervical cancer. Requiring multiple doses for vaccination often leads to poor compliance and efficacy. Recently, researchers at University of California San Diego used PLGA-FITC (AV002) as part of developing a single-dose HPV vaccine. The PLGA-FITC was used in critical studies to determine localization and fate of the delivery system as this fluorescent polymer can be easily observed under microscope as it glows brightly when illuminated with ultraviolet light. This research holds promise to reduce the incidence of cervical cancer by preventing HPV spread. Read more: Shao, Shuai, Oscar A Ortega-Rivera, Sayoni Ray, Jonathan K Pokorski, and Nicole F Steinmetz. "A Scalable Manufacturing Approach to Single Dose Vaccination against HPV." Vaccines 9, no. 1 (2021): 66. https://www.mdpi.com/966550
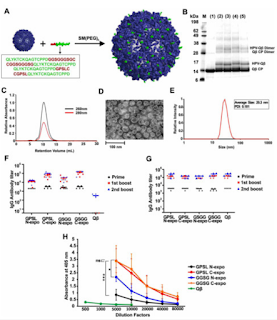
“Human papillomavirus (HPV) is a globally prevalent sexually-transmitted pathogen, responsible for most cases of cervical cancer. HPV vaccination rates remain suboptimal, partly due to the need for multiple doses, leading to a lack of compliance and incomplete protection. To address the drawbacks of current HPV vaccines, we used a scalable manufacturing process to prepare implantable polymer–protein blends for single-administration with sustained delivery. Peptide epitopes from HPV16 capsid protein L2 were conjugated to the virus-like particles derived from bacteriophage Qβ, to enhance their immunogenicity. The HPV-Qβ particles were then encapsulated into poly(lactic-co-glycolic acid) (PLGA) implants, using a benchtop melt-processing system. The implants facilitated the slow and sustained release of HPV-Qβ particles without the loss of nanoparticle integrity, during high temperature melt processing. Mice vaccinated with the implants generated IgG titers comparable to the traditional soluble injections and achieved protection in a pseudovirus neutralization assay. HPV-Qβ implants offer a new vaccination platform; because the melt-processing is so versatile, the technology offers the opportunity for massive upscale into any geometric form factor. Notably, microneedle patches would allow for self-administration in the absence of a healthcare professional, within the developing world. The Qβ technology is highly adaptable, allowing the production of vaccine candidates and their delivery devices for multiple strains or types of viruses. Keywords: HPV vaccine candidate; L2 protein; Qβ; virus-like particles (VLPs); PLGA implants; vaccine delivery device; hot melt extrusion”
No comments:
Post a Comment