Heart
disease, typically due to atherosclerotic lesions, is one of the leading causes
of death in USA. Most treatments for this disease focus on surgical interventions
(e.g. stent placement), which is often utilized in acute situations, or on
systemic medicines such as statins, which are typically applied as a
preventative. There is a need for therapies to be applied in non-emergency
situations but where atherosclerotic lesions are known to be present. Conventionally,
nanoparticles have been applied for use against cancer, however they can be
targeted to lesions by using appropriate targeting moieties. Recently,
researchers working jointly at Harvard Medical School, New York University, Technical
University of Denmark, Korea Institute of Ceramic Engineering and Technology, Korea
Advanced Institute of Science and Technology, and King Abdulaziz University
used PLA-NH2 (PolyVivo AI041, www.polyscitech.com)
from PolySciTech as a reactive precursor for generating a fluorescently-conjugated
tracer as part of a novel nanoparticle-based system for treatment of
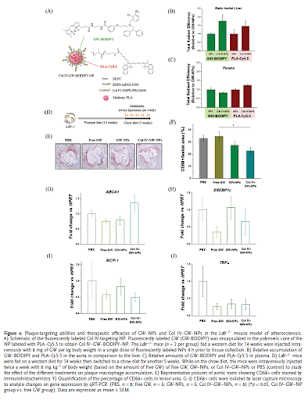
artherosclerosis. Read more: Yu, Mikyung, Jaume Amengual, Arjun Menon, Nazila
Kamaly, Felix Zhou, Xiaoding Xu, Phei Er Saw et al. "Targeted
Nanotherapeutics Encapsulating Liver X Receptor Agonist GW3965 Enhance
Antiatherogenic Effects without Adverse Effects on Hepatic Lipid Metabolism in
Ldlr−/− Mice." Advanced Healthcare Materials (2017). http://onlinelibrary.wiley.com/doi/10.1002/adhm.201700313/full
Blog dedicated to answering technical questions in an open format relating to PolySciTech (A division of Akina, Inc.) products.
Tuesday, July 25, 2017
PLA-amine from PolySciTech used in development of atherosclerosis-targeted nanoparticles for treatment of heart disease
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment